Abstract
Introduction: CD19-targeted CAR-T cells are highly active in B-cell malignancies. While early use of corticosteroids (CS) and tocilizumab have improved the management of cytokine release syndrome (CRS), CS are the mainstay of ICANS management. Though patients with low-grade ICANS often do not require therapy, severe ICANS that does not respond to CS is life-threatening. Rimiducid (RIM) is a dimerizing drug that induces apoptosis in cells engineered with an inducible caspase-9 switch. We reported the first case of the administration of a potentially ablative dose of RIM (0.4 mg/kg) to a patient with B-lymphoblastic leukemia (B-ALL) with severe, prolonged ICANS (Foster MC et al. Blood 2021 Jun 10;137(23):3306-3309). Given this patient's rapid resolution of steroid-nonresponsive ICANS and reduction in iC9 CAR.19 numbers, we sought to explore the effects of lower, potentially non-ablative doses of RIM in patients with CS-nonresponsive ICANS before the development of life threatening complications, with a goal of clinical resolution of ICANS without ablation of CAR T cells. Here, we report the clinical and pharmacodynamic courses of ICANS for the four patients treated with RIM for ICANS to date, in an ongoing expansion cohort of a phase I/II study of iC9 CAR.19 cells in patients with B-ALL.
Methods: Adults with B-ALL in 2nd or greater bone marrow (BM) relapse, relapse >100 days after allogeneic stem cell transplant, disease refractory to ≥2 induction therapies, or with measurable residual disease (MRD) persistence/recurrence were enrolled. T-lymphocytes were collected from the subjects, and CAR-T cell products generated by gene modification with a g-retroviral vector encoding for iC9, ΔNGFR (for selection and tracking purposes) and CAR.CD19 (encoding 4-1BB) genes (Diaconu I et al Mol Ther 2017; 25:580). Subjects underwent lymphodepletion with fludarabine and cyclophosphamide followed by infusion of iC9 CAR.19 cells at one of two dose levels (DL1: 5 x 105 CAR-T cells/kg; DL2: 1 x 106 CAR-T cells/kg). Toxicities were graded by CTCAE v5 or ASBMT consensus grading for CRS and ICANS. CAR-T cell expansion in peripheral blood (PB) was measured by flow cytometry (FC) and droplet digital PCR (ddPCR). Cytokines were measured by commercially available multiplex (Luminex®) cytokine assays. Leukemia response was determined by NCCN criteria. Subjects who experienced grade 3-4 ICANS despite CS for >72 hours received RIM 0.4 mg/kg IV over 2 hours. After protocol amendment, subjects with ≥ grade 2 ICANS for >24 hours despite CS received RIM 0.1 mg/kg IV over 2 hours. FC, ddPCR and cytokine levels were measured prior to RIM, at 4 hours after the end of RIM infusion and daily until ICANS resolution.
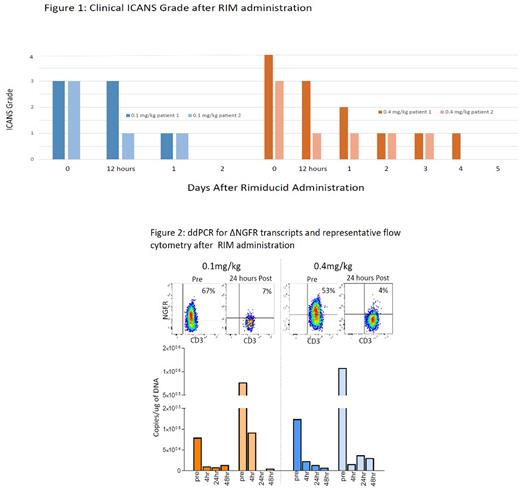
Results: 13 subjects were treated, 3 on DL1 and 10 on DL2, which was the expansion cohort cell dose. Median age was 31 years (range 21-69). CRS developed in 11, with median duration of 5 days (range 1-8). Maximum CRS grade was 2 in 5 and 1 in 6. ICANS developed in 5 subjects, with maximum grade 4 in 2, and grade 3 in 3. One patient with CS-responsive grade 3 ICANS did not meet criteria for RIM. Two subjects with grade 3-4 ICANS for >72 hours received RIM 0.4 mg/kg, both achieving improvement by at least 1 grade within 12 hours and with resolution within 5 days (FIGURE 1). ddPCR for ΔNGFR showed reduction in transcripts by 4 hours after the end of RIM infusion (by 83.6% and 98.6% respectively, FIGURE 2). In two subjects with grade 3 ICANS for 24 hours treated with RIM 0.1 mg/kg, ICANS improved to grade 1 in 24 hours and grade 0 in 48 hours (FIGURE 1). In these subjects, ddPCR for ΔNGFR showed reduction in transcripts at 4 hours after end of RIM infusion by 88.6% and 88.1% respectively (FIGURE 2). Rates of overall response (CR+CRi+MLFS at 4 weeks) were 3/4 RIM subjects versus 9/9 in other subjects. The subject with grade 3 ICANS who did not receive RIM demonstrated peak ΔNGFR transcripts, IL-6, TNF-α and IFNγ two weeks after iC9 CAR.19 infusion, whereas peak transcripts and cytokine levels occurred at one week, and prior to RIM for RIM-treated subjects.
Conclusion: RIM administration to patients with ICANS is associated with abrupt reduction of circulating iC9 CAR.19 cells and ICANS grade. Despite detection of CAR-T cells at 3 weeks post infusion and the observance of remissions after treatment with RIM, further investigation of the effects of RIM on iC9 CAR.19 persistence and antileukemic activity is warranted. Lower, potentially less ablative doses of RIM are being explored.
Disclosures
Foster:LOXO Oncology: Research Funding; Macrogenics: Research Funding; Newave Pharmaceuticals: Research Funding; Rafael Pharmaceuticals: Research Funding; Zentalis Pharmaceuticals: Consultancy; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bellicum Pharmaceuticals: Research Funding. Savoldo:Tessa Therapeutics: Consultancy. Grover:ADC: Other: Advisory Board; Kite: Other: Advisory Board; Novartis: Consultancy; Tessa Therapeutics: Consultancy; Genentech: Research Funding. Morrison:Vesselon: Consultancy. Serody:STING activation: Patents & Royalties: provisional patent to enhance CAR therapy for solid tumors/ provisional patent for the use of ILC2 cells to treat or prevent GvHD. Dotti:Bellicum Pharmaceuticals: Consultancy; Catamaran: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal